UX Blog
Timely insights on UX topics of interest

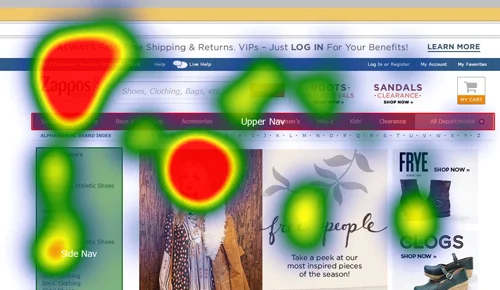
When it comes to creating products that resonate with users, usability testing plays a critical role. But once you’ve decided to evaluate your product’s usability, another question arises—should you conduct this testing remotely or in person? Each method has its unique advantages and drawbacks, and the right choice depends on your specific project requirements. This article will guide you through the pros and cons of remote and in-person usability testing, key factors to consider when making your choice, and tips for success in both approaches.

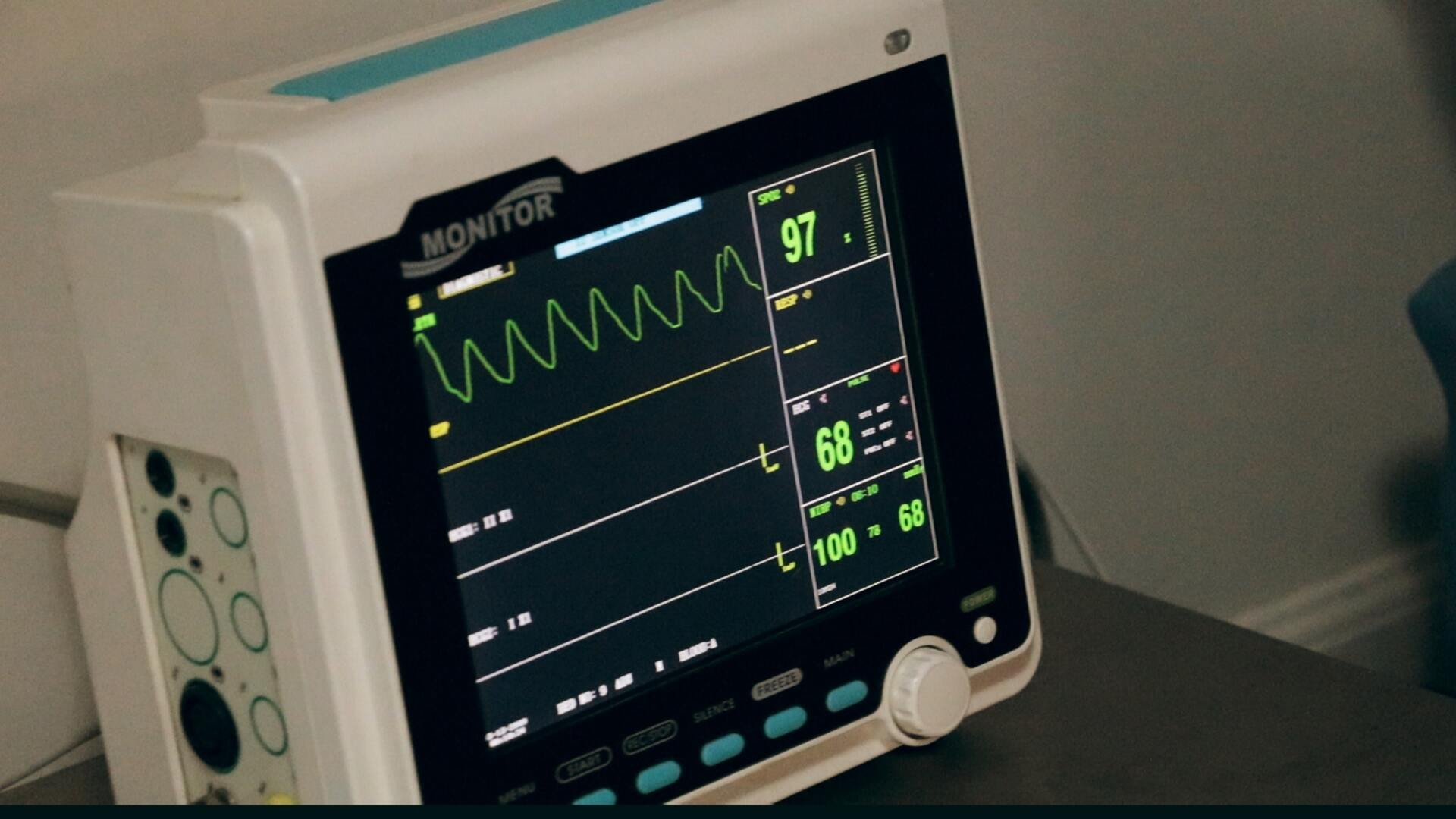
Navigating the regulatory landscape of medical devices can be a challenging endeavor. For companies aiming to bring innovative solutions to market, meeting FDA regulatory requirements is a critical milestone. But as anyone in the industry knows, even a minor misstep can lead to delays, increased costs, or even product rejection. One of the most effective ways to avoid these setbacks? Incorporate usability testing into your development process. Usability testing not only ensures that your device meets user needs but also helps identify and mitigate risks that could raise red flags during FDA reviews. Here’s why usability testing is essential and how it can safeguard your path to approval.