Medical Device Usability Testing
Our small, senior-level team comprises the top researchers in our field
Usability Testing of Medical Devices
Medical device human factors validation testing requires strict procedures following the Guidelines issued by FDA and IEC to validate the safe and effective use of medical devices. UX Firm has a proven track record of delivering effective test protocols for FDA review, high quality formative and summative usability evaluations, and highly detailed reports to be used in FDA pre-market submissions for 510(k) and De Novo classifications. We also follow European MDR and MHRA requirements and provide research that supports CE marking.
The big companies charge very big bucks for these services. Because we are a boutique consultancy, we can be highly competitive in pricing and highly effective in working directly with you to produce the research results you require.
For every medical device usability testing project, our expert 2-person team comprises the top UX researchers in our field. We partner with your team to plan the study, execute the study with your specific user groups, analyze the findings to determine root causes for all critical and non-critical tasks, and document the findings in a report according to FDA guidelines.
As part of our engagement with clients, we can do any or all of the following tasks in compliance with FDA usability guidance:
- Write the test protocol, including the moderator's guide, screener, and logging template
- Manage participant recruitment with an approved screener
- Conduct formative and summative human factors testing
- Write a comprehensive report to include root cause analysis of errors, close calls, and difficulties
Formative Usability Testing
- Engage with stakeholders to plan the study
- Determine users, tasks, environments
- Plan study tasks based on critical risk analysis
- Conduct testing and analysis
- Report findings and commend changes
FDA Human Factors Validation Testing
- Engage with stakeholders to plan study and develop test protocol
- Use uFMEA/URRA to identify critical risk factors for procedural and knowledge tasks
- Conduct testing to determine root causes of errors, close calls, difficulties
- Prepare formal report of findings, following Table A-1, Section 8, of FDA Guideline
- Conduct stakeholders findings meeting
UX Firm ensures compliance in FDA Medical Device Human Factors Validation Testing
UX Firm provides our team of highly qualified, experienced researchers to conduct formative and summative usability testing of medical devices. Our processes ensure compliance with all regulations and guidelines in support of successful FDA 510(k) and De Novo submissions.
IEC 62366, ISO 14971, & ANSI/AAMI HE75 Require Human Factors Usability Testing
IEC 62366 Part 1: Application of Usability Engineering to Medical Devices
“IEC 62366 specifies a process for a manufacturer to analyze, specify, develop, and validate the usability of a medical device as it relates to safety. . . . Part 1 has been updated to include contemporary concepts of usability engineering, while also streamlining the process.
It strengthens links to ISO 14971. . . . Part 2 [offers] more detailed descriptions of usability engineering methods that can be applied generally to medical devices.”
ANSI/AAMI HE75:2009/(R)2018 Human Factors Engineering—Design of Medical Devices
provides comprehensive guidance on designing to accommodate user characteristics, managing the risk of use error, and undergoing usability testing to confirm the safety and effectiveness of the design. This document complements ANSI/AAMI/IEC 62366.
FDA Guidance for Applying Human Factors and Usability Engineering to Medical Devices
The primary guidance was issued on February 3, 2016.
“FDA has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to maximize the likelihood that new medical devices will be safe and effective for the intended users, uses and use environments.”
The latest draft guidance was issued on December 9, 2022. It contains important information about the URRA (Use-Related Risk Analysis) table they recommend for 510(k) submissions.
"The main factors to consider in a risk-based approach to human factors assessment, as described in this draft guidance, include the identification of (i.e., presence of or modification to) critical tasks and the elimination or reduction in use-related hazards."

Read our viewpoint article on The Importance of Human Factors Validation Testing for New Medical Devices to learn the points you should consider when selecting a vendor.
“UX Firm has been a great partner to help us prepare the Usability study for our FDA submission. They have helped us in all the steps of the process, always open for discussion and giving great feedback on our product and its testing. I’m very happy that we trusted in UX Firm and I’m sure we will continue to do so in our future developments.”
"I had the pleasure of working side-by-side with Carol during a somewhat long-phased summative testing project for a medical device coming to market. Not only did UX Firm deliver, they were also flexible, knowledgeable, and a phenomenal catalyst for the project’s success. Without Carol’s expertise and guidance, we would not have been able to pass validation. UX Firm is a top-notch research firm and comes highly recommended."
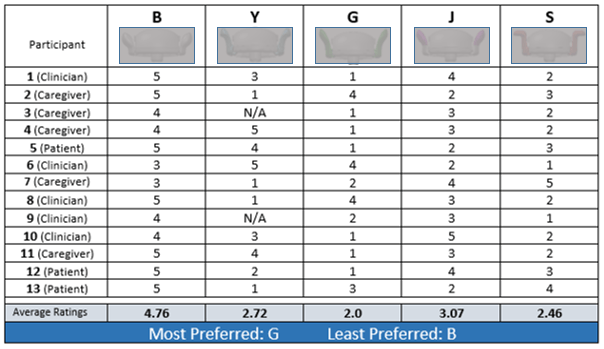
Medical Device Human Factors Case Study Example
Study Goals
Gain insights into user experience of multiple product design prototypes to determine preferences, success/failure rates, and safety of use by caregiver, clinician, and patient populations.
Methodology
Structure a comparative usability evaluation of five prototypes with representative users from three population groups, using all versions of the prototype (in varied order) to determine human factors issues, including safety and reliability, as measured against a specific set of criteria.
Outcome
A sample page from the report shows the results of one aspect of this testing. Two prototypes were determined to be the most successful and acceptable to the three user groups, with features from a third prototype reflecting some interest and acceptance from study participants.
Our recommendation was accepted to continue design and development of two prototypes to include the best features identified from this evaluation.
Retest took place in an iterative design process to determine improvements in success/acceptance in usability testing of more advanced models.