UX Blog
Timely insights on UX topics of interest

Navigating the regulatory landscape of medical devices can be a challenging endeavor. For companies aiming to bring innovative solutions to market, meeting FDA regulatory requirements is a critical milestone. But as anyone in the industry knows, even a minor misstep can lead to delays, increased costs, or even product rejection. One of the most effective ways to avoid these setbacks? Incorporate usability testing into your development process. Usability testing not only ensures that your device meets user needs but also helps identify and mitigate risks that could raise red flags during FDA reviews. Here’s why usability testing is essential and how it can safeguard your path to approval.

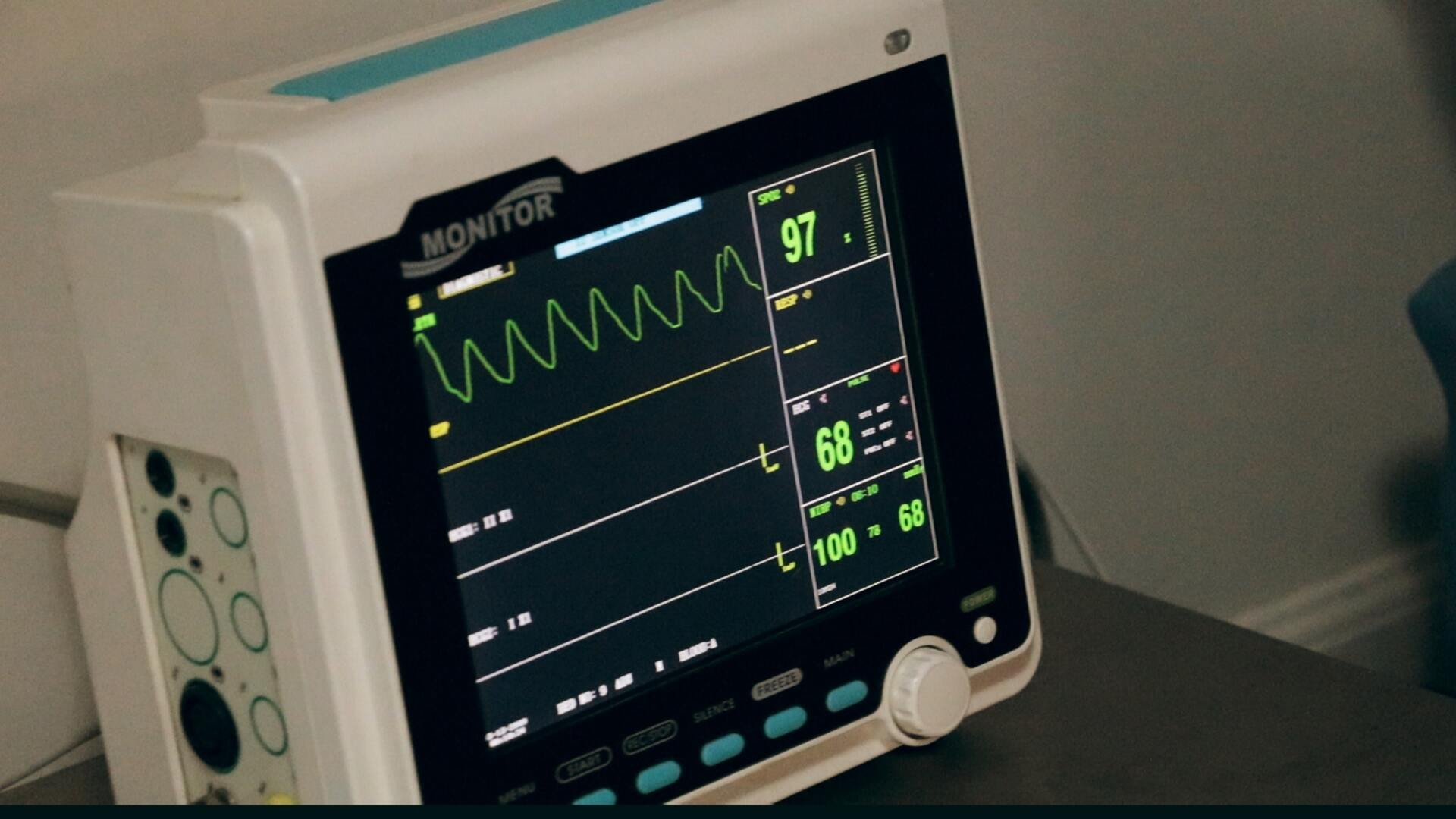
What Is human factors validation? Human factors validation focuses on assessing how intended users interact with a medical device under expected conditions in simulated use. The FDA requires this process to confirm that the device can be used safely and effectively by the intended users without causing harm or errors. It’s not about making assumptions—validation involves carefully designed usability tests where actual users perform critical tasks with the device. Their performance and any issues that arise form the basis for confirming that the device can be used safely and effectively by the intended users or if improvements are needed to the product’s design. If an iterative process has been used leading up to summative human factors validation testing, the results are more likely to confirm that users can safely use the device.